SAN DIEGO — In another study evaluating treatments for severe hair loss, Janus kinase (JAK) inhibitors have been shown to be clinically effective, according to new data presented at the American Academy of Dermatology (AAD) 2024 Annual Meeting. It was revealed that this resulted in significant hair growth.
One study of brepocitinib looked at cicatricial alopecia (CA), a hair loss condition for which there is no approved treatment. The other is a subanalysis of a phase 3 trial of retrecitinib in alopecia areata (AA), in a subset of patients enrolled in the study with alopecia totalis or alopecia universalis (AT/AU). Hair growth was shown.
Reflecting comments from multiple experts, including April W. Armstrong, MD, MPH, professor and chief of dermatology at the University of California, Los Angeles, who was one of the moderators of the belated session, the CA study He said it was consistent with the reaction. The findings of the CA biomarker study suggested breakthrough potential.
“This is the first placebo-controlled study with an oral JAK inhibitor, not only to show that cicatricial alopecia is reversible, but also to provide insight into the mechanism of action and which patients respond best. ,” said Emma Gutman Yassky, MD. said in an interview. Guttman Yassky, professor of dermatology and immunology and director of the Inflammatory Skin Disease Laboratory at the Icahn School of Medicine at Mount Sinai in New York City, was a senior investigator on the study.
Scarring alopecia and brepocitinib
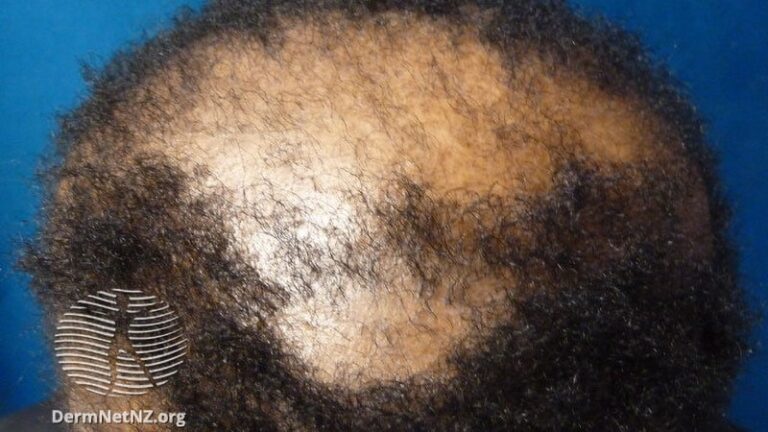
In the scarring alopecia study, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Due to the small size of the study, the primary endpoint was change in CA biomarkers. The secondary outcome was clinical response, and the two were correlated and therefore reinforced each other.
Of the subtypes, 9 patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) type alopecia, and 24 had central centrifugal cicatricial alopecia. The patient was suffering from inflammatory syndrome (CCCA). All forms of CA are common among women in general, women of color in particular, and CCCA in particular. In this analysis, FFA and LPP subtypes were considered similar and were combined to assess response.
Data include response and safety comparisons during the 24-week randomization phase, as well as additional follow-up conducted after 24 weeks of open-label treatment. In the second phase, all patients receiving placebo were switched to active treatment.
Overall, all four of the major scalp inflammatory biomarkers measured were reduced in the FFA/LLP combination group. In the placebo group, each marker, including interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1, increased over the same period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern was observed for CCLS and CXCL10 (increase in the placebo group and decrease in the brepocitinib group). For IFN-gamma and STAT1, increases were observed between the placebo and active treatment groups, but the increases for placebo were greater.
In terms of clinical response, improvements in disease activity index with brepocitinib were observed, particularly among patients in the FFA/LLP group, said Dr. Marguerite Maryman, resident dermatologist at Mount Sinai who presented the results. She said the improvement in clinical activity scores at week 48 was “dramatic.” Additionally, improvement was evident within 4 weeks of starting treatment.
For CCCA, which is a more difficult condition to treat, further progression may be an acceptable response for many patients, although there have been cases of hair regrowth in this subset, Maryman said. . Although she did not see the improvement typically seen in FFA/LLP patients, she suggested that there is room for improvement even in these more difficult patients.
Further research is planned, but Professor Maryman said it may be important to focus on early treatment, regardless of the subtype of CA. He noted that patients who have had the disease for less than five years usually have better outcomes than those who have had the disease for longer.
Light lecitinib for AT/AU
The analysis of AT/AU patients was based on a subset analysis of the ALLEGRO phase 2b/3 trial of litrecitinib, which targets JAK3 and TEC kinases. Full results from the ALLEGRO trial were published last year in The Lancet.
In the new late-stage analysis, the Severity of Alopecia Tool (SALT) score was assessed on an observation basis or on a carryover basis from previous observations. In general, the response of the subgroup of AT/AU patients with a median SALT score of 80.3 at baseline (meaning 80.3% hair loss) was slightly lower than the overall study response.
At 24 months, about 50% of patients achieved a SALT score of 20, said Melissa Pillian, M.D., director of dermatology at the Cleveland Clinic in Cleveland, Ohio, who presented the data. In this group, similar to the non-AT/AU population, responses increased over time, and these responses were maintained as long as patients continued to receive treatment.
At a more stringent threshold of SALT < 10, the proportion of responders was slightly lower, but this is a truly excellent result, as a significant proportion of AT/AU patients achieve ``90% or greater hair regrowth.'' "It's a reaction," Pirian said.
A significant proportion of eyebrow and eyelash regeneration was also observed, particularly in the AU subgroup, Piliang said. Because hair loss often carries a devastating psychological burden, patient-reported global ratings of these responses were generally “even better” than researchers-reported ratings.
However, Piliang advised clinicians to treat AA as soon as possible. Despite the benefits seen in the AT/AU subgroup, starting treatment before complete hair loss increases the likelihood of complete or near-complete hair regrowth, he noted.
There are no data from the ALLEGRO trial to determine how long hair regrowth lasts after discontinuation of litrecitinib, which is approved for the treatment of AA, but lifelong treatment should be expected for the majority of people. Pirian said patients should be told this. , whether AA has advanced to AT/AU.
“My experience with JAK inhibitors is that when you stop these drugs, you lose the response,” she says.
Maryman and Pirian spoke during the late break session of the American Academy of Dermatology (AAD) 2024 Annual Meeting on March 9 in San Diego, California.
Mr. Maryman reported financial relationships with AbbVie. Mr. Pillyan reported financial relationships with Eli Lilly, Pfizer, and Procter & Gamble. Mr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including manufacturers of JAK inhibitors. Mr. Guttmann Yassky reported financial relationships with more than 30 companies, including manufacturers of JAK inhibitors.
Ted Bosworth is a medical journalist based in New York City.